Page number 173
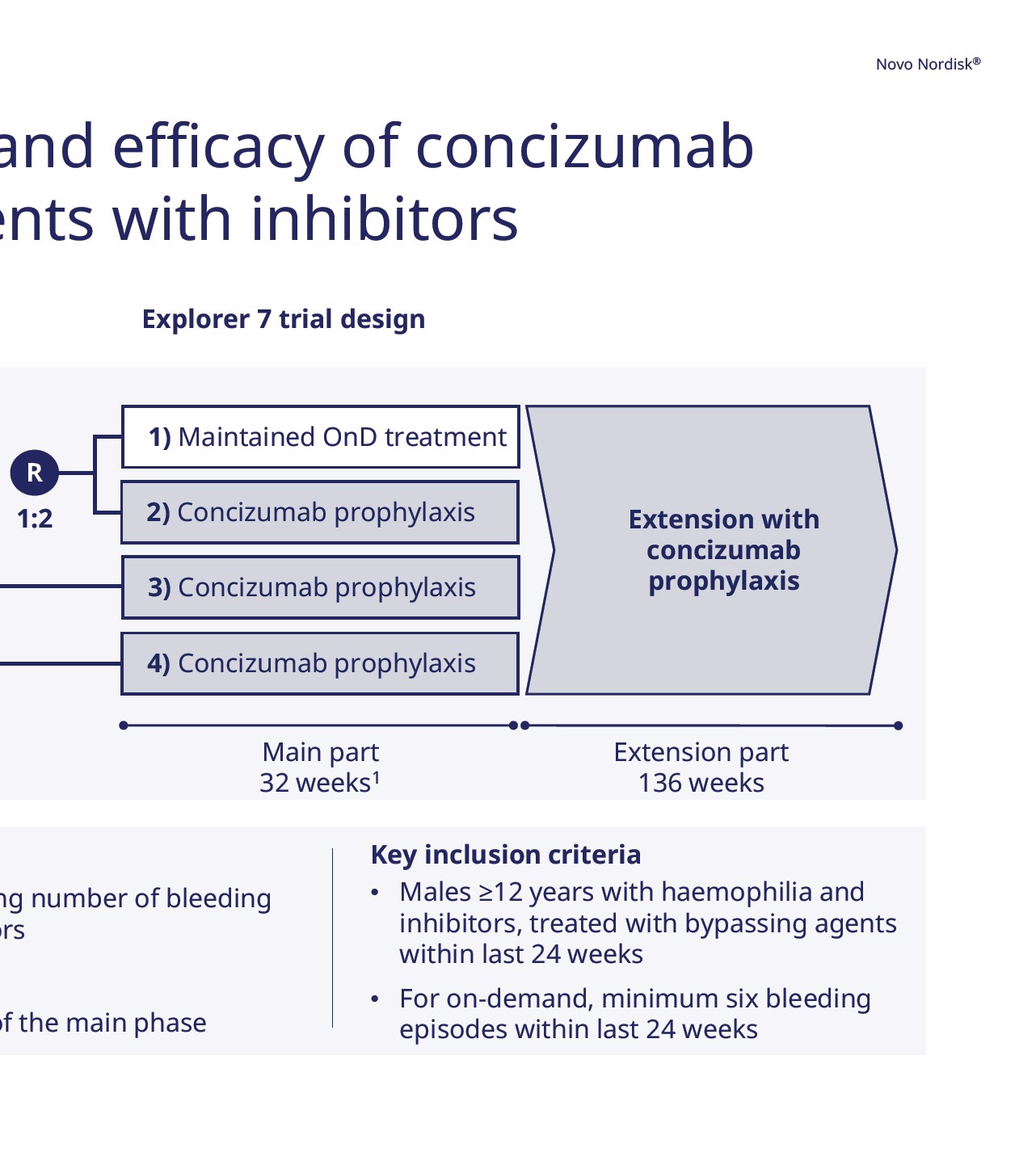
87 Investor presentation First six months of 2023 In the Explorer 7 trial, concizuma in adults and adolescents with in Explorer 7 trial results: Annualised bleeding rate per patient group 100 Median Mean Annualised Bleeding Rate (ABR) 90 40 30 20 10 9.8 0 0 0 0 OnD treatment PPX treatment PPX treatment PPX treatment HwI HwI HAwI HBwI (Group 1) (Group 2) (Groups 1-4) (Groups 1-4) Primary endpoint Note: The box represents Q1-Q3 (25 th to 75 th percentile). Whiskers are 5 th and 95 th percentile. HA: Haemophilia A; HB: Haemophilia B; HAwI: Haemophilia A with inhibitors, HBwI: Haemophilia B with inhibitors; HwI: Haemophilia
Page number 174
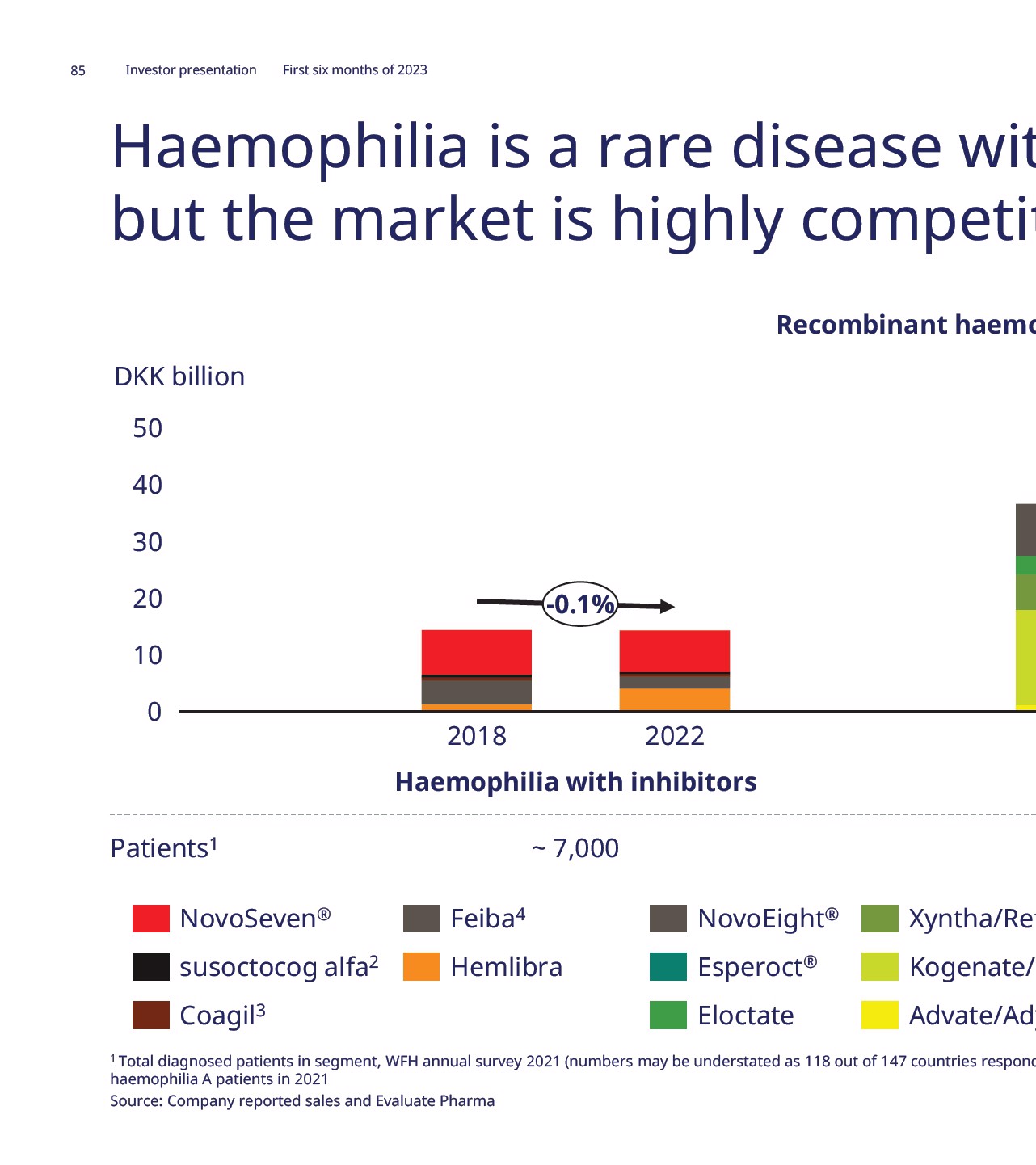
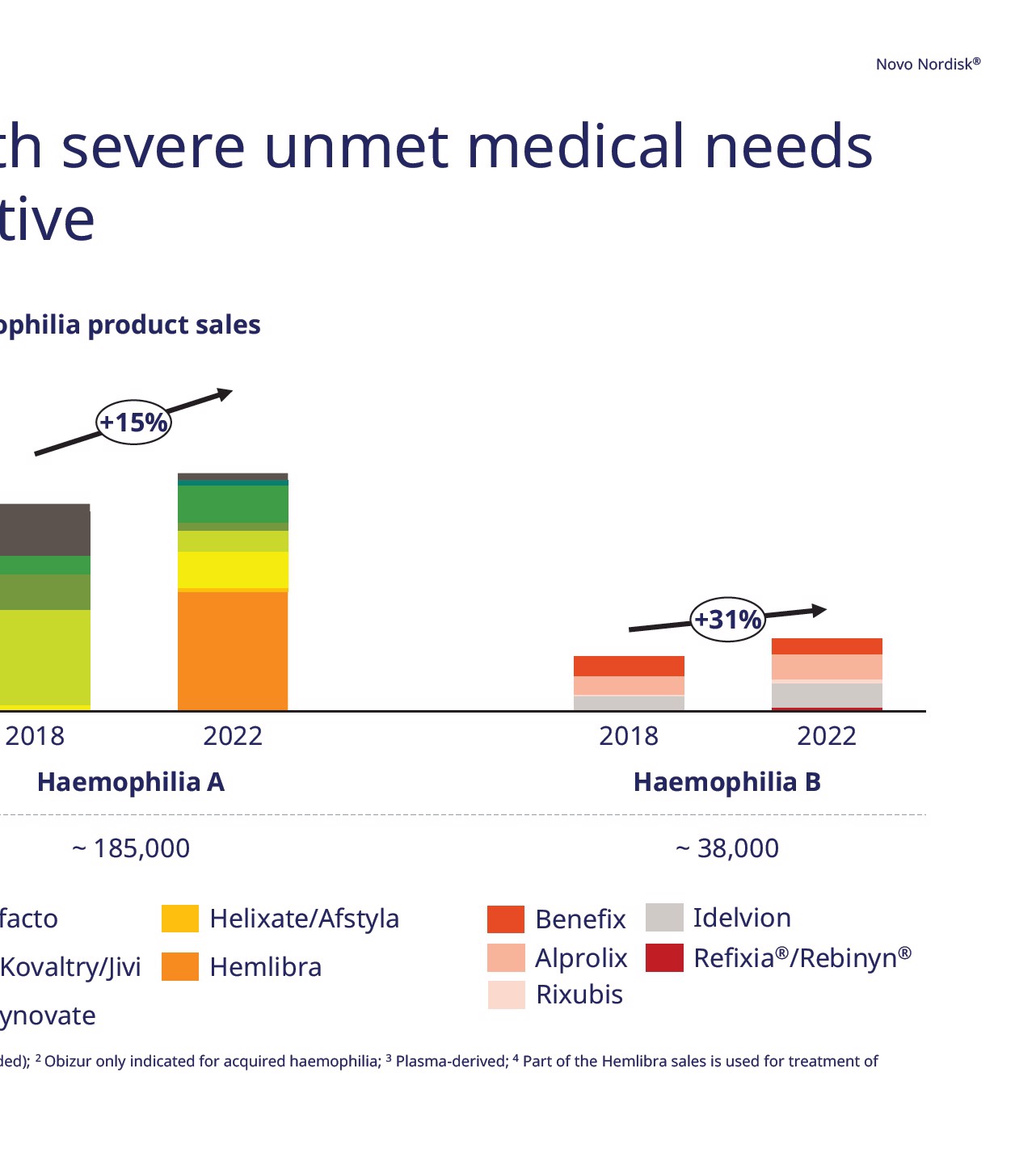
Novo Nordisk ® ab reduced the number of bleeds nhibitors Key highlights Efficacy • Median ABR was 0 for concizumab prophylaxis treatment, compared to 9.8 in the on-demand treatment group • Estimated mean ABR was 1.7 for concizumab prophylaxis treatment, compared to 11.8 in the on-demand treatment group • For patients on concizumab prophylaxis, 64% had 0 bleeds in Group 2 Safety • Concizumab appeared to have a safe and well tolerated profile Status • US Complete Response Letter for HwI received in Q2 2023, resubmission end of 2023 expected • JP submission for HwI completed in Q3 2022 • Explorer8 in non-inhibitor patients was completed in Q3 2022 a with inhibitors; OnD: On-demand; PPX: Prophylaxis; ABR annualised bleeding rate